Distillation Under Reduced Pressure Is Used to Purify Liquids Which
Such liquids are made to boil at a temperature lower than their normal boiling points by reducing the pressure on their surface. Distillation under reduced pressure.

What Is The Difference Between Distillation Distillation Under Reduced Pressure And Steam Distillation
This is done under lowered pressure levels and generally involves the distillate traveling a very small distance before being collected hence the.

. If the boiling point of water is increased when the external pressure is increased then decreasing the external pressure should decrease the boiling point. Steam Distillation is dependent on vapour pressure. This technique is applied for the purification and separation of high boiling liquids or liquids which decompose partially or completely at or below their normal boiling points.
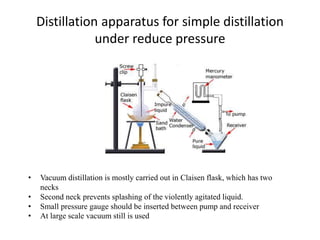
Glycerol is purified by distillation under reduced pressure. Glycerol at normal pressure the bp. Short path distillation is used to purify a small quantity of a compound that is unstable at high temperatures.
How to Purify by Distillation at Reduced Pressures Vacuum Distillation How it works. Since the boiling points of liquids are depressed at reduced pressure organic compounds can be distilled at lower temperatures. Method of distillation under reduced pressure is used to purify liquids havi Assertion.
A liquid boils when its vapour pressure is equal to the atmospheric pressure. Complete step by step answer. As you know the boiling point of a liquid occurs when its vapor pressure is equal to the external pressure.
This technique must be used if the liquid boils above about 150 degrees Celsius where it is likely to decompose. Steam Distillation is used to purify the impure liquid by passing steam and is applicable under specific conditions only. A liquid boils when its vapour pressure becomes equal to the external pressure.
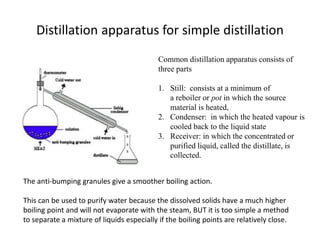
This method is used for the purification of high boiling liquids and liquids which decompose at or below their boiling points. Distillation under reduced pressure is used to purify a liquid that has a habit of decomposing on boiling. The apparatus for distillation is shown.
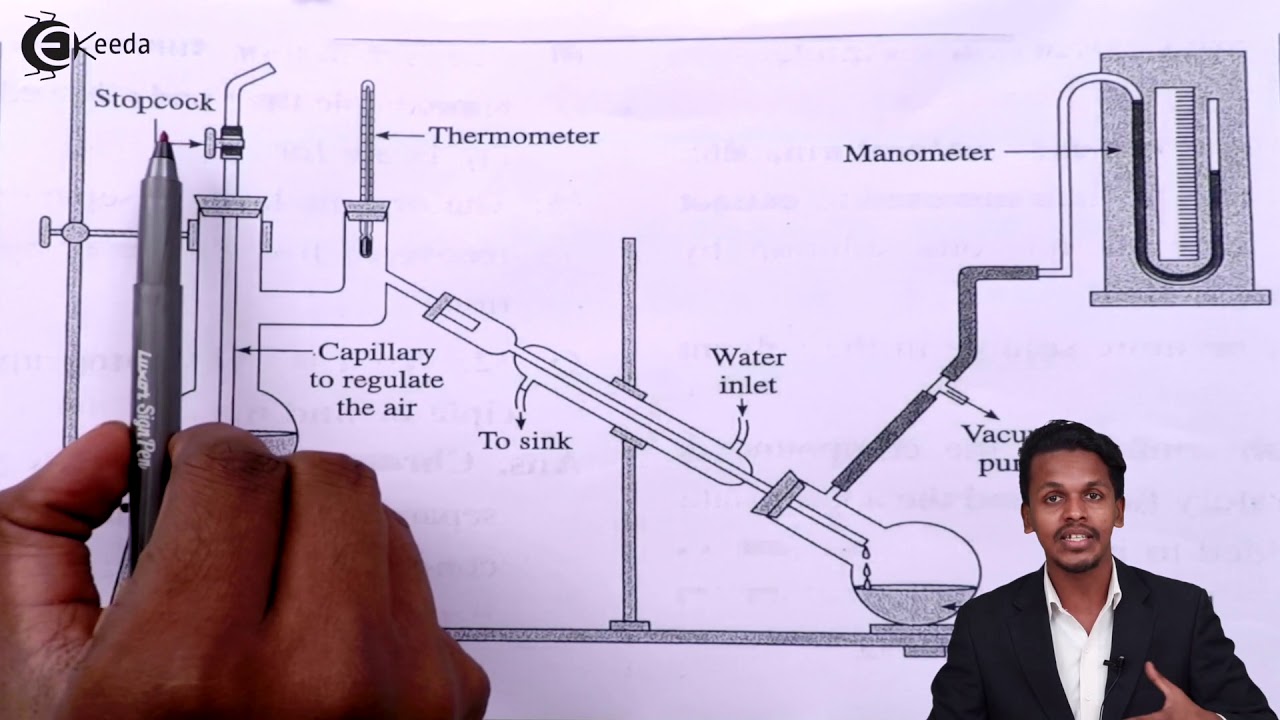
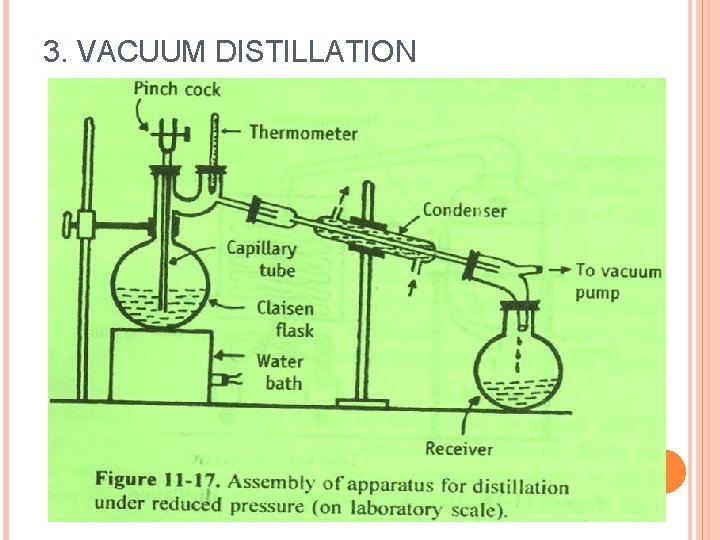
It is done to distil a compound before it gets decomposed. The principle behind distillation under reduced pressure is that a liquid reaches its boiling point when its vapour pressure is equal to the atmospheric pressure. Claisen flask having two necks.
The apparatus used for distillation under reduced pressure is shown in figure. However it illustrates an important principle that is used in the distillation of many materials. At the conditions of the reduced pressure the liquid will boil at a lower temperature than its boiling point and therefore the liquids will not decompose.
Of glycerol is 563 K but it decomposes on this temperature. Distillation is a process of separating the component substances from a liquid mixture by selective evaporation and condensation. Lowering of pressure on the surface of a liquid lowers its boiling point.
-Distillation is the process of separating different components of a mixture on the basis of difference in their boiling points. If a system is under vacuum it has a reduced pressure and the. It is fitted with a long drawn jet dipping in the liquid to be distilled.
Distillation under reduced pressure is used to purify liquids having very high boiling points and those which decompose at or below their boiling points. While this is not particularly important for the purification of water this principle is used in the process of freeze drying an important. At the conditions of the reduced pressure the liquid will boil at a lower temperature than its boiling point and therefore the liquids will not decompose.
Distillation under reduced pressure. Distillation under reduced pressure also involves conversion of a liquid into vapours by heating followed by condensation of the vapours thus produced by cooling but the pressure acting on the system is not atmospheric but is reduced by using a vacuum pump. This process of distillation under reduced pressure is known as vacuum distillation.
Distillation under reduced pressure It is used to purify liquids having very high boiling points and those which decompose at or below their boiling point. This method is used to purify liquids having very high boiling points and those which decompose at or below their boiling points. Since the boiling point of a liquid decreases as the pressure acting on it is reduced therefore this method is used to.
Distillation Under Reduced Pressure or Vacuum Distillation. If both assertion and reason are true and reason is the correct explanation of assertion. It is one of the most common laboratory techniques used by chemists for the purification and identification of organic liquids.
When the pressure is reduced by suction the liquid boils at a lower temperature. Because different compounds often have different boiling points the components will separate from a mixture. Distillation under reduced pressure Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor pressure usually less than atmospheric pressure causing evaporation of the most volatile liquids those with the lowest boiling point.
A reduced-pressure vacuum distillation is performed at a reduced pressure using a water aspirator or a mechanical pump. -Distillation is used for purification of alcohol crude oil refining desalination etc. The only difference between steam distillation and normal distillation is that along with the substance to be distilled steam is also used in the distilling jar.
Vacuum distillation or distillation under reduced pressure is used to separate liquids that have very high boiling points or those compounds which decompose just before or at their boiling point. Distillation under reduced pressure is used to purify a liquid that has a habit of decomposing on boiling. Distillation under reduced pressure This technique is used for purifying or separating thermally unstable liquid compounds which decompose at their normal boiling points.
The third type of distillation is a vacuum distillation.

Distillation Under Reduced Pressure Is Generally Used To Purify Those Liquid Which

Purification Of Organic Compounds Methods Of Purification Of Liquids
Purification Distillation At Reduced Pressures

What Is The Difference Between Distillation Distillation Under Reduced Pressure And Steam Distillation
Purification Distillation At Reduced Pressures
Distillation Process Pharmaceutics

What Is The Difference Between Distillation Distillation Under Reduced Pressure And Steam Distillation
By Distillation Under Reduced Pressure
Distillation Definition Distillation Is An Unit Operation Which

Which Of The Following Method Is Used To Purify Liquids Having Very Highboiling Points And Those Which Decompose At Or Below Their Boiling Points A Simple Distillation B Fractional Distillation C Distillation Under Reduced Pressure D

Distillation Under Reduced Pressure Is Generally Used To Purify Those Liquid Which

Distillation Under Reduce Pressure Purification Of Organic Compound Class 11 Chemistry Youtube

Distillation Process Pharmaceutics

Purification Of Organic Compounds Types Methods Principles Videos

What Types Of Liquids Are Purified By Distillation Under Reduced Pressure What Is Its Principle From Chemistry Organic Chemistry Some Basic Principles And Techniques Class 11 Cbse
Purification Fractional Distillation
What Are The Five Advantages Of Carrying Out Distillation Under Reduced Pressure Quora

Comments
Post a Comment